
by
Wayne, Pa. (August 14, 2019) — The latest pharmaceutical unit-dose package capability and new films for medical device applications will be featured by Tekni-Plex’s Tekni-Films business unit at Pack Expo Las Vegas, September 23-25, Las Vegas Convention Center, Booth N206.
As a result of Tekni-Plex’s recent Lameplast acquisition, the company will be discussing semi-automatic and fully automatic Pentafill™ fill/seal machines for its injection molded unit-dose packaging. This two-step technology, represented in the United States by Lameplast’s LF of America subsidiary, provides a benefit-rich alternative to the traditional one-step, blow/fill/seal approach.
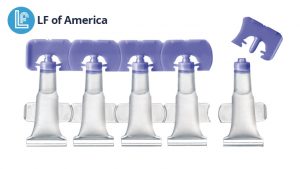 With the Lameplast system, fillers receive pre-made vials that provide a higher level of precision, functionality and quality control compared to blow/fill/seal. Because they are injection molded, instead of blow molded, the vials offer a more attractive physical appearance (no burrs), uniform orifice/drug delivery, flexible closure design and printing capability, ability to injection mold with barrier materials vs. only one material, more uniform wall thickness, and reclosability. These are all attributes that are not possible when the unit dose vial is blown, filled and sealed in one stage. Applications include pharmaceutical, diagnostic, medical device, veterinary and cosmetic applications, with an emphasis on ophthalmic, vaccine, vaginal and rectal applications.
With the Lameplast system, fillers receive pre-made vials that provide a higher level of precision, functionality and quality control compared to blow/fill/seal. Because they are injection molded, instead of blow molded, the vials offer a more attractive physical appearance (no burrs), uniform orifice/drug delivery, flexible closure design and printing capability, ability to injection mold with barrier materials vs. only one material, more uniform wall thickness, and reclosability. These are all attributes that are not possible when the unit dose vial is blown, filled and sealed in one stage. Applications include pharmaceutical, diagnostic, medical device, veterinary and cosmetic applications, with an emphasis on ophthalmic, vaccine, vaginal and rectal applications.
Tekni-Films will also be featuring TekniMD™ PX films, a series of proprietary high-performance, thermoformable copolyester films that provide an alternative to PETG for medical device packaging. Standard PX film is suitable for form, fill and seal applications, while PX MED has superior denesting characteristics and is used as a substitute for silicone-coated PETG.
Additionally, Tekni-Films will be discussing its SBC 240 film line as a performance and improved-cost alternative for 4- and 6-mil PCTFE laminations and cold formed foil for blister applications. SBC is created by applying a next generation, high-barrier PVdC coating to a lamination of PVC and polyethylene. The PVdC is dispersed in an emulsion, which has been engineered as a surface coating.
This thermoformable film has exceptional moisture and oxygen barrier properties and is designed to meet the needs of pharmaceutical and other healthcare applications. The triplex structure is ideal for applications such as pharmaceuticals, nutraceuticals, probiotics and other related products.
About Tekni-Plex, Inc.
Tekni-Plex is a globally-integrated company focused on developing and manufacturing products for a wide variety of end markets, including medical, pharmaceutical, food, beverage, personal care, household and industrial. Tekni-Plex is headquartered in Wayne, PA, and operates manufacturing sites across nine countries worldwide to meet the needs of its global customers. For more information visit www.tekni-plex.com.


